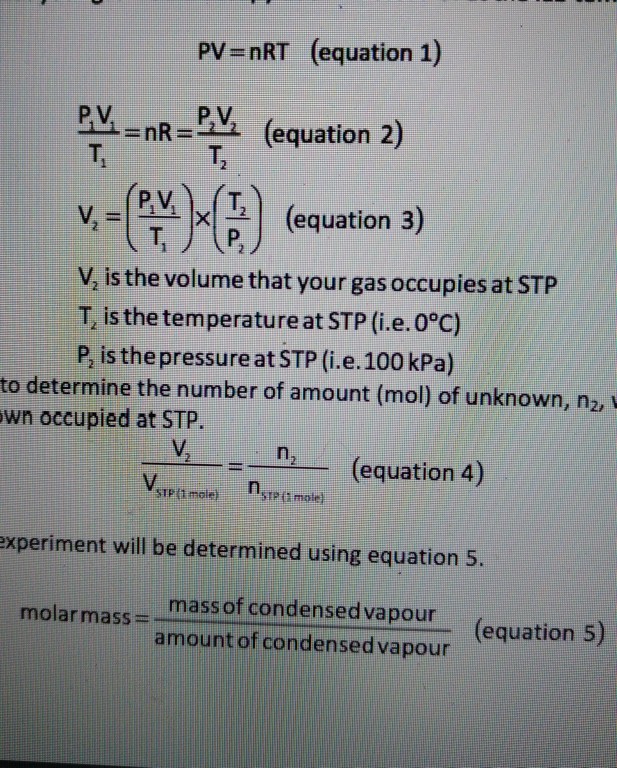
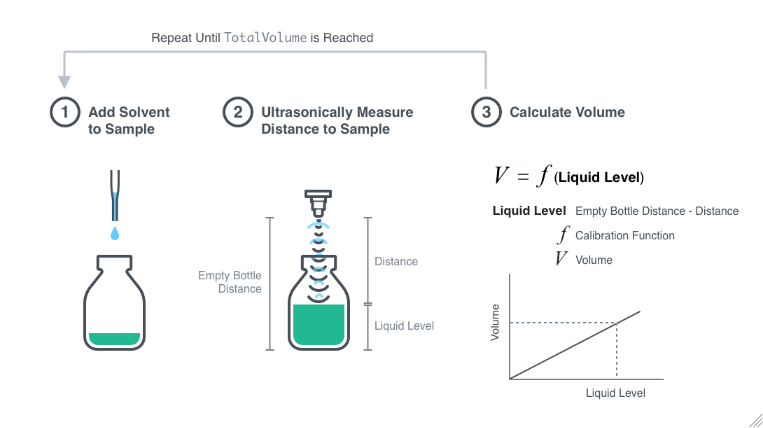
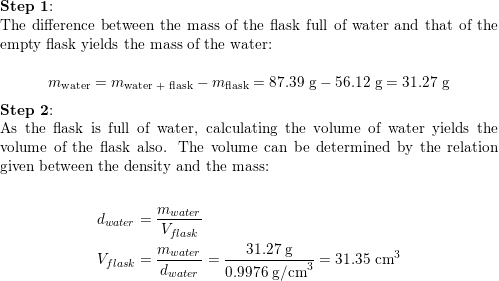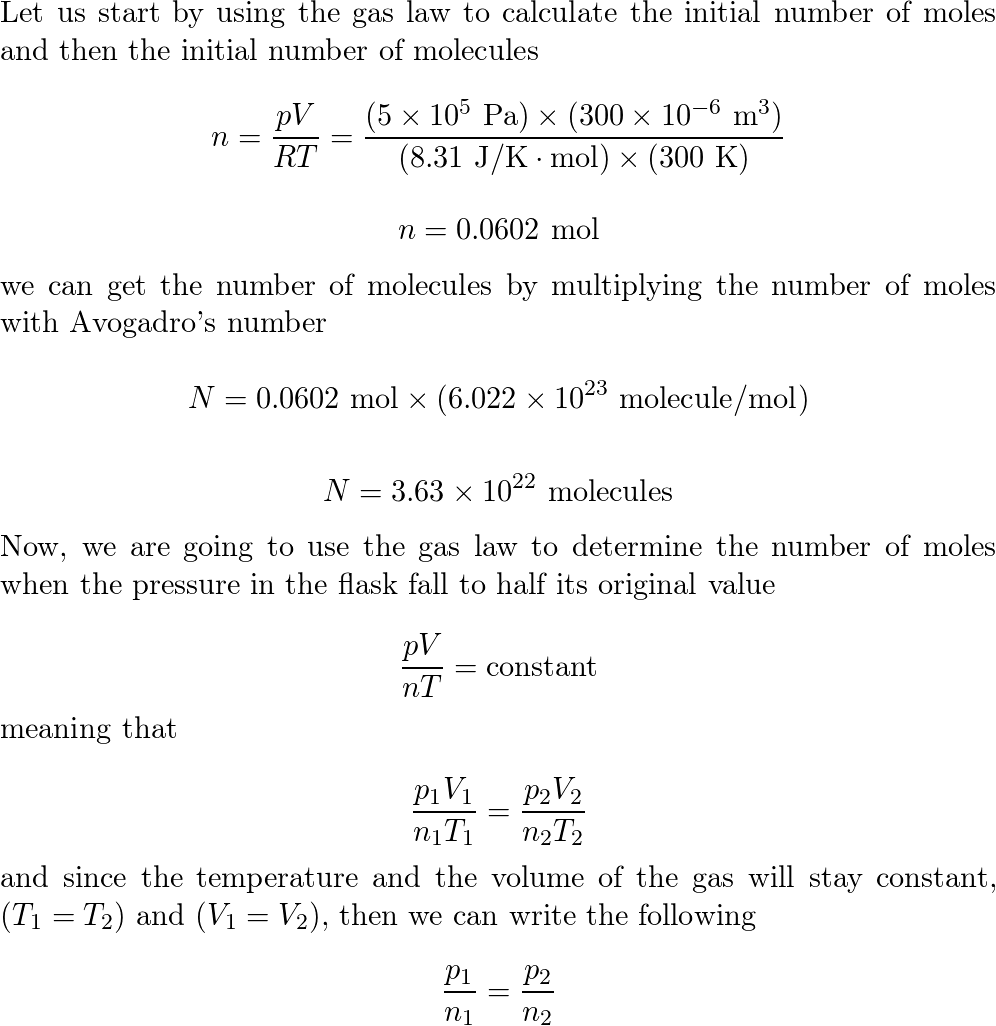SOLVED:An empty Erlenmeyer flask weighs 241.3 g. When filled with water (d=1.00 g / cm^3), the flask and its contents weigh 489.1 g . (a) What is the flask's volume? (b) How

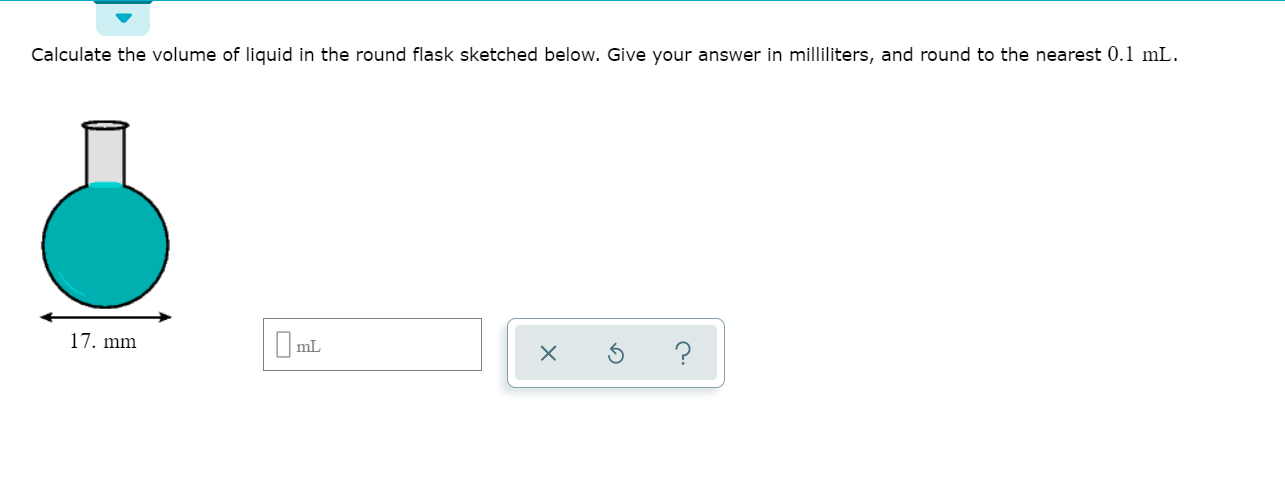
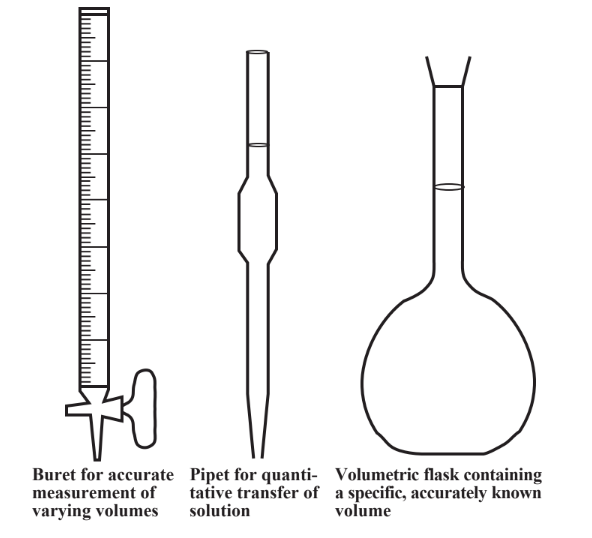
SOLVED:Finding the volume of a flask. A student obtained a clean dry glass-stoppered flask. She weighed the flask and stopper on an analytical balance and found the total mass to be 31.601
0.01 mole of mehane and 0.96 g of oxygen were enclosed in a flask maintained at a temperature 300k.The pressure inside the flask was found to be 101325 nm.Calculate the volume of
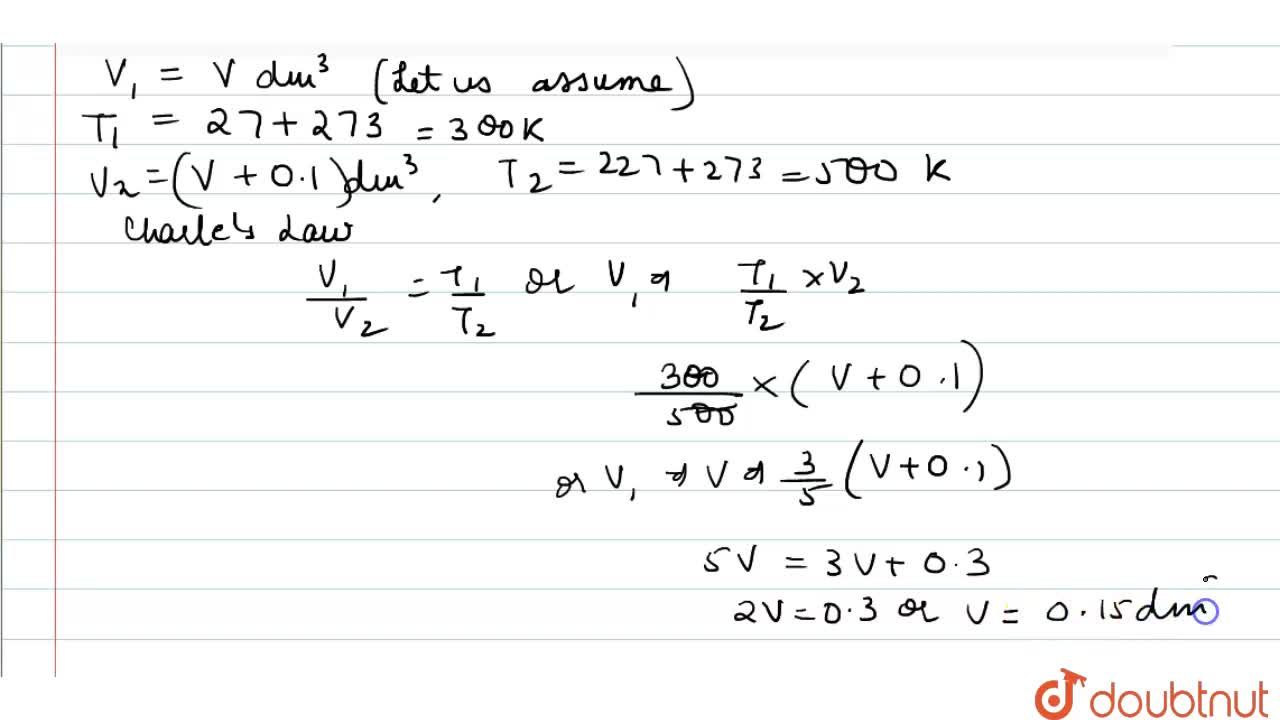
A flask was heated from 27^(@)C to 227^(@)C at constant pressure. Calculate the volume of the flask if 0.1 dm^(3) of air measured at 27^(@)C was expelled from the flask.

A glass flask of volume one litre at 0^o C is filled level full of mercury at this temperature. The flask and mercury are now heated to 100^o C. How much mercury