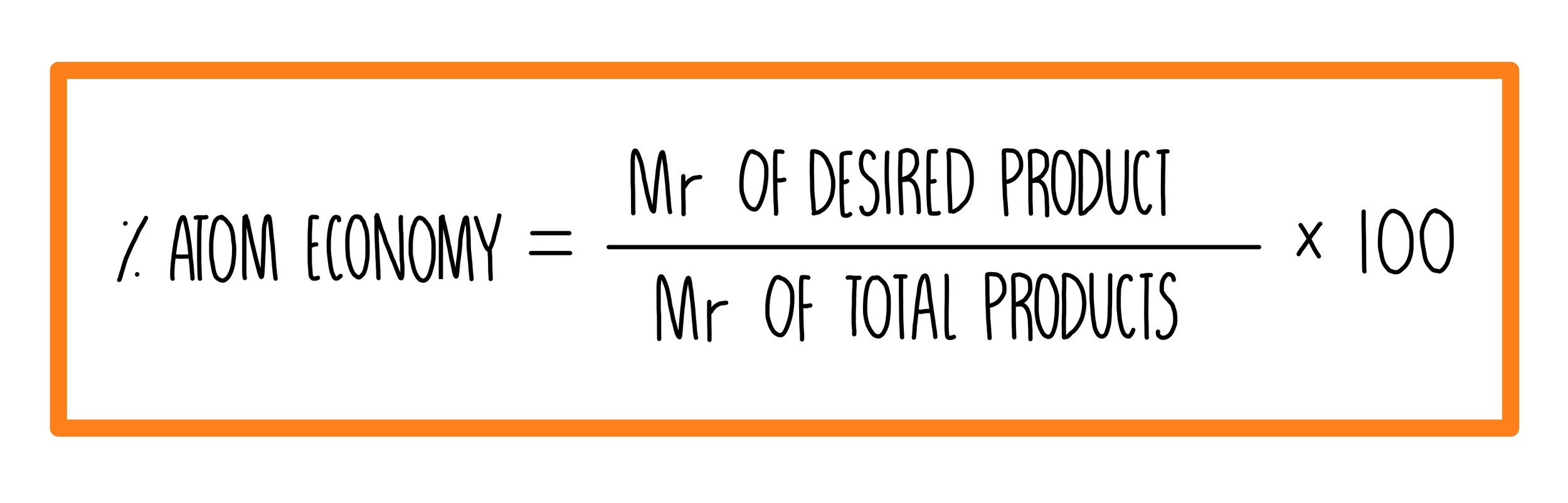
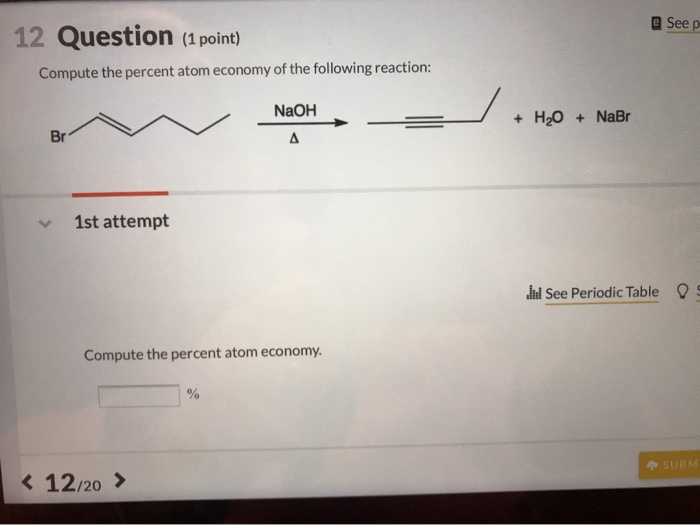
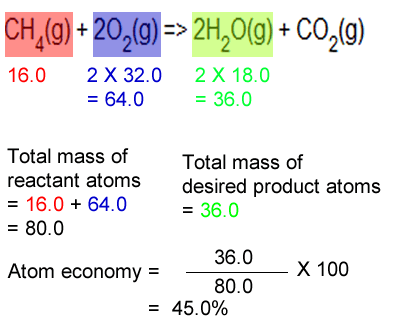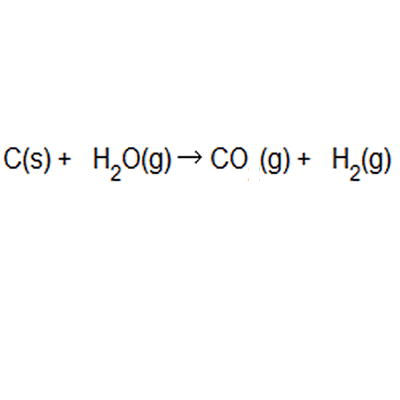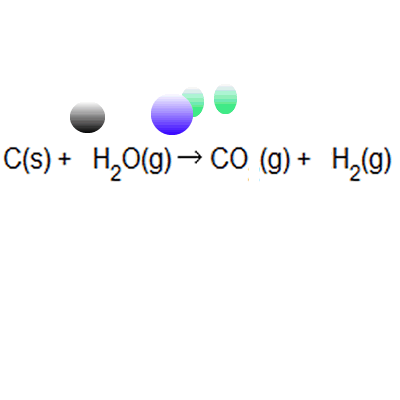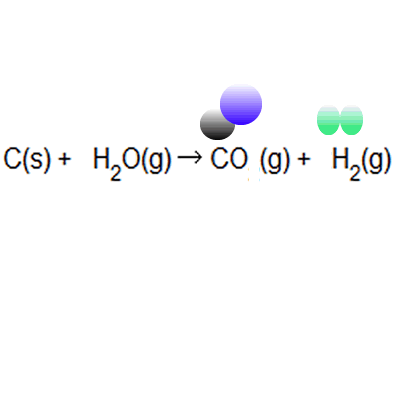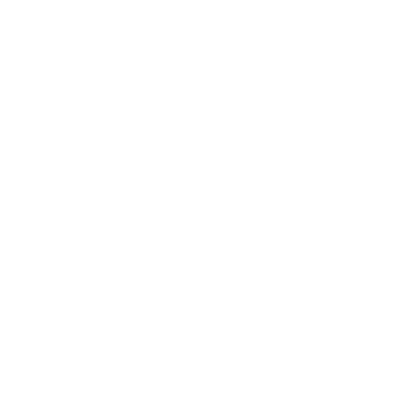Calculating Percent Composition and Determining Empirical Formulas - Video & Lesson Transcript | Study.com
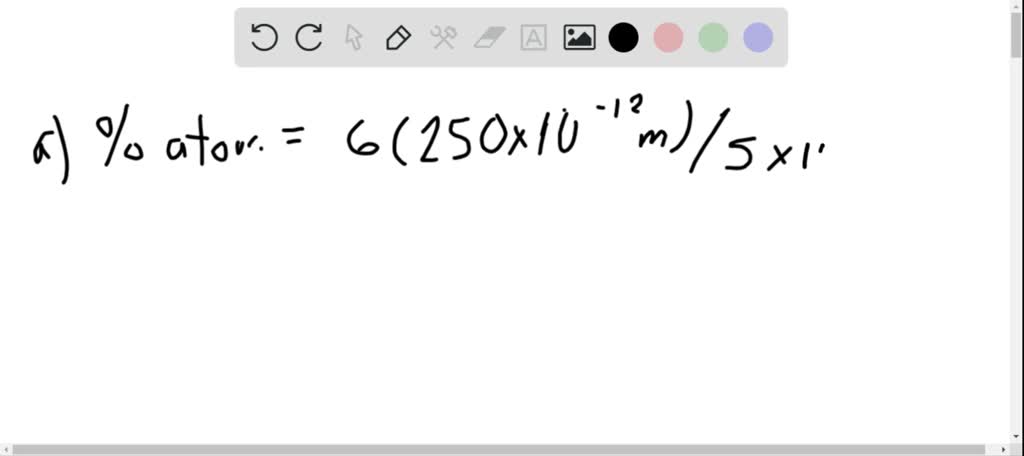
SOLVED:Calculate the percentage of the atoms that are on the surface of a cubic nanoparticle if the diameter of the atoms is 250 pm and the edge length of the particle is (