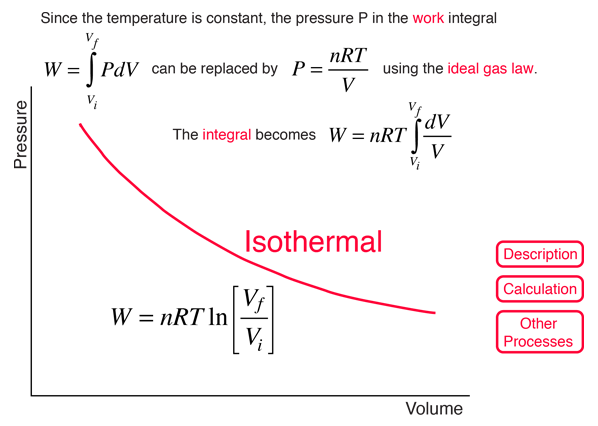
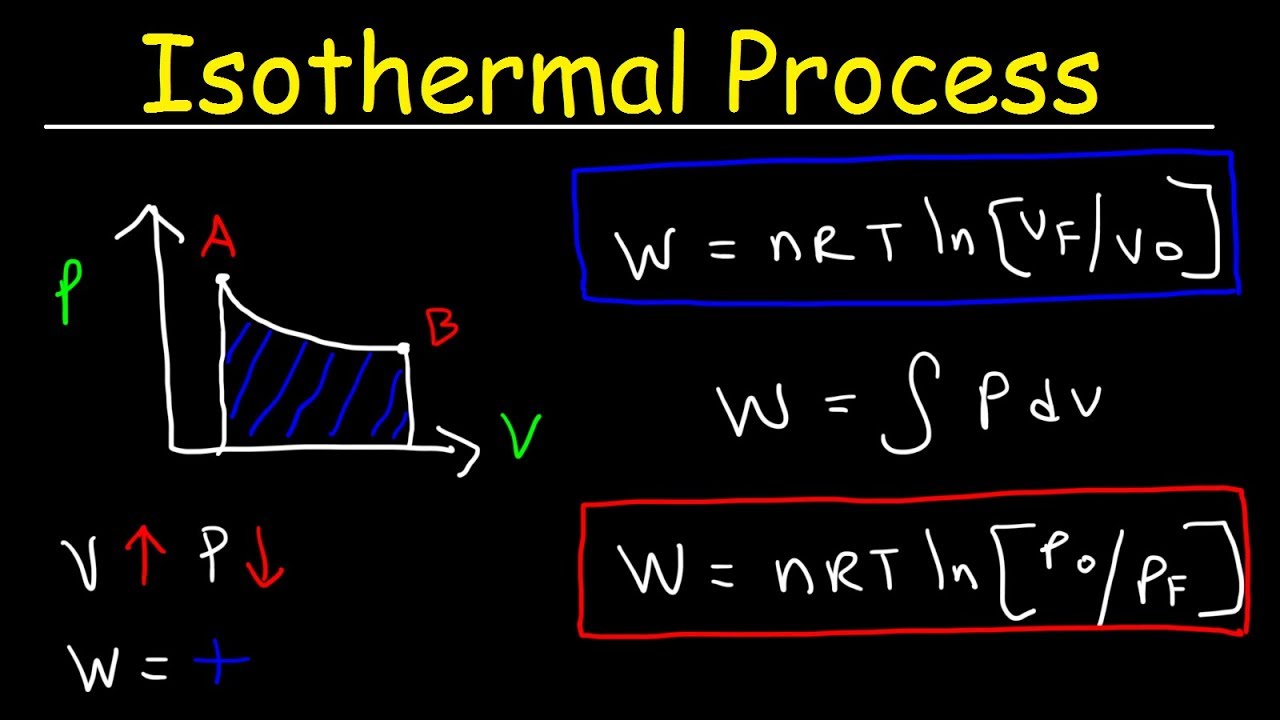
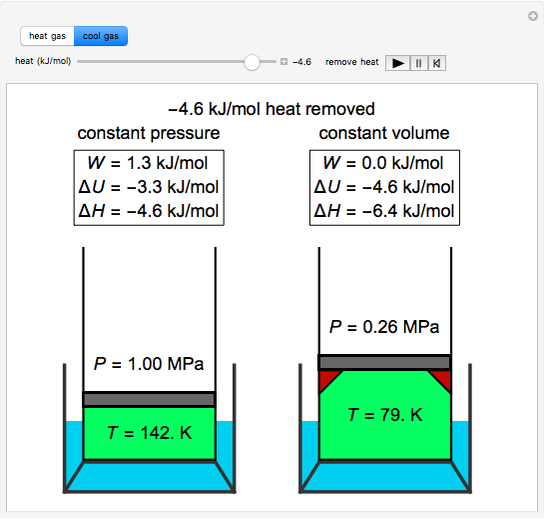
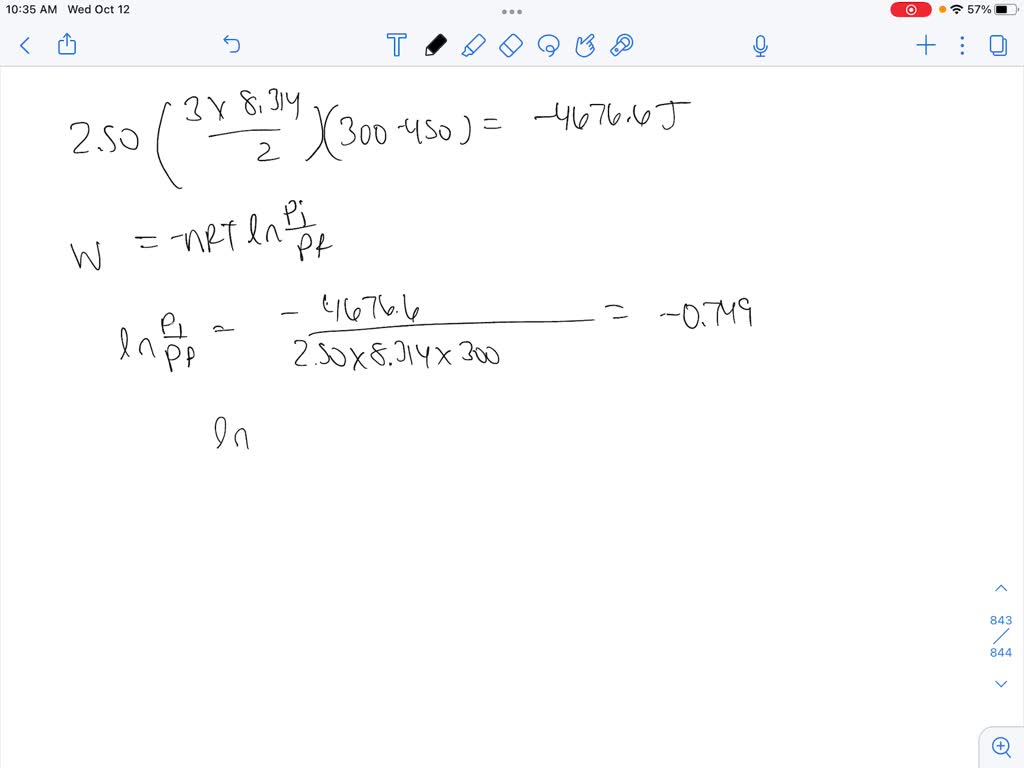How is the final temperature of irreversible adiabatic expansion of an ideal gas calculator? - Quora

Calculate the final temperature of a monoatomic idal gas that is compressed reversible and adiabatic - YouTube
✓ Solved: A sample of an ideal gas at 15.0 atm and 10.0 L is allowed to expand against a constant external...

Fig. S 6: Gas expansion factor as a function of calculated centreline... | Download Scientific Diagram

A gas expands from `3 dm^(3)` to `5 dm^(3)` against a constant pressure of 3 atm. The work done - YouTube

SOLVED:A sample of nitrogen gas expands in volume from 1.6 to 5.4 L at constant temperature. Calculate the work done in joules if the gas expands (a) against a vacuum, (b) against

homework and exercises - How do you calculate the change in temperature of an adiabatic system? - Physics Stack Exchange

Methane Gas Volume Expansion Ratios and Ideal Gas Deviation Factors for the Deep-Water Bering Sea Basins: Peng-Robinson Equation of State